
Standard ambient conditions are generally taken as temperature 25oC or 298.15 K, and pressure 1 bar or 105 pascals or 0.987 atm.
Whether a mineral is stable in any particular environment may depend on several factors, including pressure, temperature,
acidity, whether the environment is oxidising or reducing and the concentration of different components which may affect
alteration reactions. Stability diagrams maybe drawn showing the changes with respect to the two most important variables,
keeping other possible variables at a constant value.
Common types of stability diagrams are
For example the stability ranges for the three paramorphs of
Al2OSiO4, kyanite,
andalusite and sillimanite

Standard ambient conditions are generally taken as temperature 25oC or 298.15 K, and pressure 1 bar or
105 pascals or 0.987 atm.
Pourbaix diagrams plot Eh against pH at constant temperature of 25oC and pressure 1 bar.
pH is a measure of the acidity of the environment, that is a measure of the abundance of H+ ions; it takes values up
to 14, with a higher value indicating a
more alkaline environment (because of the way it is defined in thermodynamics, a bigger number means fewer H+ ions).
pH=1 is for an extremely acid environment, pH=7 is neutral, and pH=14 is extremely alkaline. In
natural near-surface environments pH usually ranges from about 4 to about 9 (KB p225).
Just as pH is a measure of abundance of H+ ions, so Eh corresponds to the abundance of O2-
ions, available for oxidation; Eh is a measure of the oxidation potential of the environment. Again because of
its definition in thermodynamics, possible values vary from about -0.8 volts to about +1.2 volts, with high values for
oxidising environments and low or negative values for reducing environments.
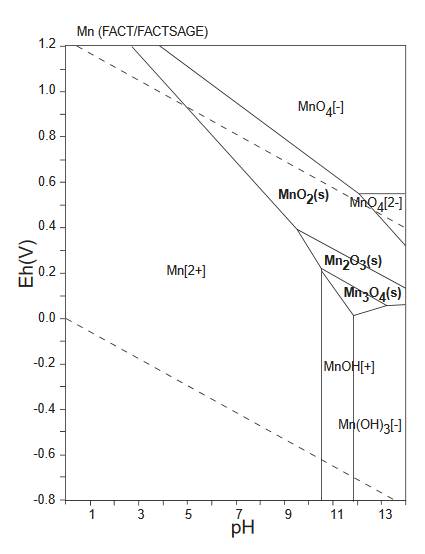
For example the diagram below is a Pourbaix diagram for manganese, created by Andel Früh and available on Wikimedia Commons.
It shows the relationship between akhtenskite MnO2, bixbyite
Mn2O3, hausmannite Mn3O4 and
pyrochroite Mn(OH)3.
The concentration of various ions in solution can affect the solubility of different minerals, and therefore the likelihood of
their formation as solid precipitates. In thermodynamics a quantity called "activity" is defined, which is closely connected with
ionic concentration. This quantity is usually given the symbol "a", and aCu2+ denotes the activity of
divalent copper ions in the solution. Because of the way in which it is defined, it is better to take the logarithm of the
activity as an indication of the concentration of ions, with a higher value of the log representing a greater concentration.
The logs are usually negative, so, for example, -2 represents a greater concentration than -5. See above for the meaning of pH.
The diagram below was calculated at 298.2 K for the main Cu2+ and Pb2+ arsenate minerals.
Boundaries are calculated for constant activities (roughly equivalent to concentrations) of Pb2+ and Cl-
in solution, over a range of values of pH and of Cu2+ activity
(LMW p269).

Back to Minerals