Smithsonite is often found as a secondary mineral in the oxidation zone of zinc ore deposits in limestone. It has also been observed in sedimentary deposits and as a direct oxidation product of sphalerite.
It is associated with sphalerite, galena, hematite, cerussite, calcite and limonite. It is often found as pseudomorphs after calcite. In the oxidation zone of epithermal veins sphalerite ZnS (primary) alters to secondary hemimorphite, smithsonite and manganese-bearing willemite.
It may form pseudomorphs after calcite (RES p148).
Localities
At the Huanggang Fe-Sn deposit, Hexigten Banner, Chifeng City, Inner Mongolia, China, pure white botryoidal smithsonite has been found, that fluoresces white under short wave UV. (AESS).
Smithsonite from Huanggang - Image
At the San Antonio mine, Chihuahua, Mexico, smithsonite pseudomorphs after calcite have been found (KL p167).
Smithsonite from San Antonio - Image
At Tsumeb, Namibia, smithsonite pseudomorphs after aragonite and after azurite have been found (KL p165, 166).
Smithsonite from Tsumeb comes in a rainbow of colours, from copper-rich (apple-green and turquoise-blue) to cobalt-rich (pink to deep rose), manganese-rich (pale pink) and cadmium-rich (yellow, possibly intensified by inclusions of greenockite), in addition to white, grey and shades of brown and black (from sulphide inclusions). It is the most abundant of the secondary zinc minerals, occurring in large masses and botryoidal crusts; rhombohedral to scalenohedral crystals can reach several centimetres in size and rank as the finest in the world. Brown to yellowish and white crystals from the top of the first oxidation zone are the least attractive, though they reached large size (6 cm), and colourless, doubly terminated crystals to 15 cm are known. The highly desired green and pink varieties were found in the second oxidation zone in the 1970s. Sharp, deep pink rhombohedrons looking more like rhodochrosite were found in the third oxidation zone. Other known associations may include malachite, wulfenite, mimetite, tennantite and germanite. (Minrec 55.6 supplement p144).
Smithsonite from Tsumeb - Image
At the Berg Aukas Mine, Grootfontein, Otjozondjupa Region, Namibia, smithsonite directly replaced sphalerite, and, microscopically, willemite often replaced smithsonite. A typical paragenesis is sphalerite - smithsonite - willemite - ferric oxides. In the orebodies smithsonite was found either as granular masses forming a solid smithsonite rock, or as vein-filling botryoidal aggregates lining or filling fissures and cavities (R&M 96.2.133-136).
At the Hudgill Burn Mine, Alston Moor, Eden, Cumbria, England, UK, rare yellow-green botryoidal smithsonite has been found (AESS).
Smithsonite from the Hudgill Burn Mine - Image
The Nelly James Mine, Miller Canyon, Miller Peak, Cochise County, Arizona, USA, is a former small surface lead, copper, zinc, gold and silver mine located at an altitude of 7250 feet. Mineralisation is a vein deposit Mindat). The mine is now famous for fluorescent minerals collected from the dumps, including calcite (fluoresces red), hydrozincite (sky blue), powellite (creamy-yellow), smithsonite (crimson red), sphalerite (yellow-orange) and willemite (green).
Smithsonite is usually grey, white or tan in daylight. It can be found massive or as colourless to tan botryoids. The honeycombed variety is also present. Under shortwave UV light the response can be a bright crimson-red, bright pink, pale blue or deep blue. Under longwave UV the response is a muted tan with medium brightness. The response under middle range UV is about the same as longwave with more of a pinkish tan colour. Some smithsonite specimens exhibit a very brief sustained luminescence upon removal of the shortwave UV source. However, the possibility exists that calcite is intergrown with this smithsonite and is the cause of this brief sustained luminescence (R&M 97.1.48-56).
Smithsonite from the Nelly James Mine - Image
At the Philadelphia mine, Rush, Marion county, Arkansas, USA, smithsonite pseudomorphs after dolomite and after sphalerite have been found (KL p168-170).
Smithsonite from the Philadelphia Mine - Image
At Cookes Peak mining district, Luna county, New Mexico, USA, smithsonite was the primary zinc ore, and is found in many places where heavy oxidation of sphalerite has occurred, usually on a limestone/gossan matrix (R&M 94.3.235-236).
Smithsonite from Cookes Peak - Image
At the Tintic Mining District, Utah, USA, smithsonite is generally abundant and single crystals are known from several mines, but they are small, only reaching a millimeter or two. It occurs much more commonly as coatings on fracture surfaces and as vein fillings, and some larger specimens of this type are known. A single specimen from an unknown mine, measuring 7 cm, is composed of a matrix of quartz with botryoidal coatings of pale blue smithsonite on fracture surfaces; many parts of this specimen are covered by druses of azurite crystals (MinRec 55.2.221).
Smithsonite from Tintic - Image
At the Kabwe mine, Central Province, Zambia, smithsonite has been found associated with tarbuttite, parahopeite or willemite (R&M 94.2.134-138).
Smithsonite from Kabwe - Image
Alteration
The first stage in the formation of zinc supergene minerals is the oxidation of sphalerite to zinc sulphate, which is very soluble and remains in solution as zinc and sulphate ions:
ZnS + 2O2 → Zn2+ + SO42-
(JRS 18.14).
hydrozincite and CO2 to smithsonite and H2O
Zn5(CO3)2(OH)6 + 3CO2 ⇌ 5ZnCO3 + 3H2O
At pH between 5 and 8.5 (somewhat acid to somewhat alkaline) either hydrozincite or smithsonite will form, depending on the availability of carbonates. If this availability changes, then hydrozincite may change to smithsonite and vice versa, according to the above equation. Increased availability of carbonates favours the forward reaction and the formation of smithsonite (JRS 15.60-61). Smithsonite is found only in oxidised ore deposits (carbonate-rich), where hydrozincite is very rare, and hydrozincite, but not smithsonite, commonly occurs as coatings on mine walls and dumps, where the carbonate concentration is lower (JRS 18.14).
The Activity-pH diagram below was calculated at 298.2 K for smithsonite, hydrozincite and adamite for constant activity (roughly equivalent to concentration) of H2AsO4- in solution, over a range of values of pH and of H2CO3 activity (MM 52.688).
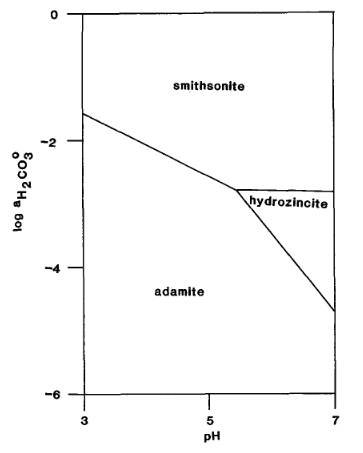
The mineral formulae are:
smithsonite: Zn(CO3)
hydrozincite: Zn5(CO3)2(OH)6
adamite: Zn2(AsO4)(OH)
Back to Minerals