For carbonate concentration slightly lower than atmospheric, cerussite and hydrocerussite can co-exist in alkaline environments, with leadhillite in more acid environments, and anglesite in yet more acid environments. At extremely low carbonate concentrations the rare mineral lanarkite can form in conditions of pH between those for anglesite and leadhillite (JRS 18.11)
Localities
At Tsumeb, Oshikoto Region, Namibia, leadhillite crystals from this locality are the largest and finest known. About 20 fine specimens were found in in a single pocket in corroded tennantite in the second oxidation zone. They range in habit from small and poorly formed to superb pseudohexagonal tablets and pyramids as large as 13 cm, and weighing several kilograms. The colour ranges from white to dark grey and pale yellow, usually opaque but sometimes partially gemmy. The softness and micaceous cleavage with pearly lustre on the cleavage face are diagnostic. Associations include melanotekite, anglesite, cerussite, kegelite and alamosite (Minrec 55.6 supplement p108).
Leadhillite from Tsumeb - Image
At Balliway Rigg, Caldbeck, Allerdale, Cumbria, England, UK, leadhillite has been found in cavities in oxidised galena in quartz veins, associated with caledonite, mattheddleite and lanarkite. It is sometimes associated with anglesite, cerussite or linarite (JRS 11.15-16).
At the Red Gill mine, Roughton Gill, Caldbeck, Allerdale, Cumbria, England, UK leadhillite occurs as crusts on quartz associated with numerous minerals including anglesite, linarite, caledonite, bindheimite, cerussite and susannite (JRS 11.38).
Leadhillite from the Red Gill Mine - Image
At Short Grain, Deer Hills, Caldbeck, Allerdale, Cumbria, England, UK leadhillite is common in the supergene post-mining assemblage, and in some specimens it replaces lanarkite. Crystals are also produced by in situ oxidation in the vein. It is often associated with its paramorphs susannite and macphersonite, and sometimes occurs with scotlandite, native silver or caledonite (JRS 12.54-55).
At Silver Gill, Roughton Gill, Caldbeck, Allerdale, Cumbria, England, UK leadhillite occurs associated with anglesite and caledonite (JRS 8(2).91-92).
At Driggith mine, Caldbeck, Allerdale, Cumbria, England, UK, on the dumps, leadhillite occurs in oxidised galena-bearing fragments associated with caledonite, anglesite and cerussite (JRS 9.23).
Leadhillite from the Driggith Mine - Image
At the Brae Fell mine, Roughton Gill, Caldbeck, Allerdale, Cumbria, England, UK, leadhillite occurs associated with anglesite, caledonite, cerussite and linarite (JRS 9.41).
At Whitwell quarry, Derbyshire, England, UK, leadhillite is associated with galena and baryte (RES p138).
Leadhillite from the Whitwell Quarry - Image
At the Manila Mine, Cochise county, Arizona, USA, leadhillite occurs associated with anglesite, and in vugs with caledonite, diaboleite, linarite and lanarkite (R&M 90.4.344).
Leadhillite from the Manila Mine - Image
At the Mammoth-St Anthony mine, Tiger, Pinal county, Arizona, USA, a specimen has been found of leadhillite on and pseudomorph after cerussite (KL p182).
Leadhillite from the Mammoth-St Anthony Mine - Image
At the Eureka Hill Mine, Eureka, Tintic Mining District, Juab County, Utah, USA, leadhillite has repotedly been found in one occurrence at the mine, associated with anglesite (MinRec 55.2.209).
At the Upper dumps, North Star Mine, Mammoth, Tintic Mining District, Juab County, Utah, USA, leadhillite has been found in several small, heavily oxidised boulders of goethite and quartz with pods of partially oxidised galena. The crystals are rather small, the largest so far noted being a single pseudo-hexagonal, transparent crystal only about 1 mm wide. Good crystals of cerussite and anglesite up to several millimetres are seen in small vugs near the oxidised galena, as well as small amounts of hemimorphite. The leadhillite occurs in these vugs, invariably associated with anglesite or cerussite (MinRec 55.2.209).
Leadhillite from the Upper Dumps - Image
Alteration
Heating leadhillite causes it to reversibly transform into its paramorph susannite in the temperature range from 50 to 82°C.
Stability
The Activity-pH diagram below was calculated for some lead minerals. Boundaries are calculated for constant activity (roughly equivalent to concentration) of (SO4)2- and constant partial pressure (also roughly equivalent to concentration) of CO2, over a range of values of pH and of Cl1- activity. In this case the concentration of CO2 is above the atmospheric value.
Both paralaurionite and leadhillite can be stable at this level of CO2 concentration, although they are not at higher levels. If the concentration of CO2 decreases further the stability field of leadhillite widens to include a greater range of pH (JRS 15.20).
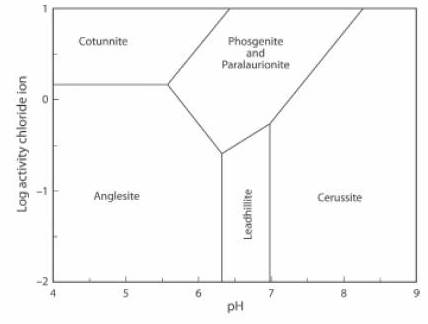
The lead mineral formulae are:
cotunnite PbCl2
phosgenite Pb2(CO3)Cl2
paralaurionite PbCl(OH)
cerussite Pb(CO3)
anglesite Pb(SO4)
leadhillite Pb4(CO3)2(OH)2
The Activity-pH diagram below is similar, but the concentration of CO2 is close to zero, at about 0.01% of the atmospheric value, and the (SO4)2- activity is about 0.5% of its value in the first diagram.
Cerussite does not form in these conditions, the stability field of mendipite is very large, that of leadhillite is small, and mereheadite and plumbonacrite can form, although they are not stable at higher levels of concentration of CO2 (JRS 15.18-23).
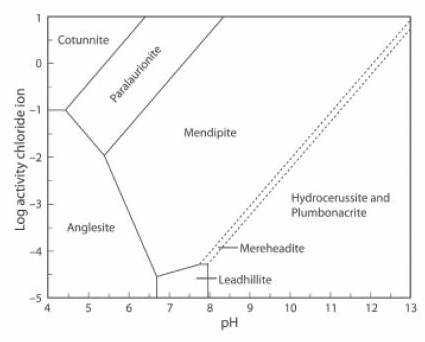
The lead mineral formulae are:
cotunnite PbCl2
paralaurionite PbCl(OH)
mendipite Pb3O2Cl2
mereheadite Pb47O24(OH)13cl25(BO3)2(CO3)
hydrocerussite Pb3(CO3)2(OH)2
plumbonacrite Pb5(CO3)3O(OH)2
anglesite Pb(SO4)
leadhillite Pb4(CO3)2(OH)2
Back to Minerals