Lead will generally precipitate as primary galena from ore fluids rich in sulphur and lead. Removal of sulphur by precipitation of sulphides, however, may lead ultimately to an ore fluid from which galena cannot be precipitated, even with a high concentration of lead in solution. In these circumstances, anglesite, as well as cerussite and pyromorphite, could be precipitated as a primary mineral. (Strens (1963), MM 33.722-3).
Anglesite is commonly associated with galena, sphalerite, smithsonite, hemimorphite and iron oxides.
Localities
At the Nakhlak Mine, Anarak District, Nain County, Isfahan Province, Iran, epigenetic (formed later than the surrounding or underlying rock formation) vein deposits and metasomatic replacement bodies are hosted by a chalky Upper Cretaceous (100.5 to 66 million years ago) limestone. The limestone underwent dolomitisation prior to sulphide mineralisation. The principal primary ore mineral is galena, associated with minor or trace amounts of sphalerite, tetrahedrite -tennantite, pyrite and chalcopyrite as inclusions. The main secondary ore mineral is cerussite, sometimes associated with minor amounts of anglesite, plattnerite, wulfenite, minium, mimetite, covellite, chalcanthite, malachite and goethite. Many trace elements are present in the primary galena, but most notably it is rich in silver and antimony and poor in bismuth.
Anglesite occurs sparingly at the Nakhlak mine, as milky white, lustrous crystals to 1 cm or more on galena matrix (Minrec 54.3.383-408).
Anglesite from the Nakhlak Mine - Image
At Tsumeb, Namibia, anglesite has been found associated with native copper (R&M 93.6.539). Also, wulfenite and mimetite pseudomorphs after anglesite have been found here (KL p216, 206).
A variety of colours and crystal habits are known, including bright yellow (cadmium-rich), green (copper-rich) and colourless to grey. The matrix, if any, is typically sulphide minerals. Associations may include willemite, smithsonite, azurite and malachite, as well as wulfenite and mimetite (Minrec 55.6.supplement p22).
Anglesite from Tsumeb - Image
At Caldbeck Fells, Cumbria, England, UK, anglesite is uncommon but widespread. At Brae Fell mine it has been found associated with cerussite, pyromorphite and galena. At the Barndy Gill lead mine it is associated with wulfenite. At the Driggith mine and at Short Grain it is associated with galena. At Dry Gill it occurs with mimetite variety campylite. At Red Gill mine it is associated with linarite or caledonite, and at Silver Gill it is occasionally found altered to leadhillite (C&S).
At Whitwell quarry, Derbyshire, England, UK, anglesite occurs on an oxidised galena - baryte matrix (RES p136, 137).
Anglesite from the Whitwell Quarry - Image
At the PC Mine, Cataract Mining District, Jefferson county, Montana, USA, anglesite occurs as a dull grey coating on galena (R&M 96.6.494).
At the Blanchard mine, Bingham, New Mexico, USA, anglesite pseudomorphs after galena have been found (KL p188).
Anglesite from the Blanchard Mine - Image
At the Tintic Mining District, East Tintic Mountains, Utah, USA, anglesite occurs as a common weathering product of galena and other lead-bearing ore minerals at many localities. One of its most common forms is massive anglesite replacements of galena. The anglesite grows along cleavage planes in galena and replaces the galena inwards, forming a boxwork texture. It can be found as small colourless crystals lining cavities in galena, as grains replacing galena, and on quartz. It can also occur as single crystals to over 1 cm and as sub-millimeter crystalline coatings on galena, quartz and goethite.
The Eureka Hill mine produced some of the better anglesite crystals, found in vugs in altered galena (MinRec 55.2.177).
Anglesite from Tintic - Image
Alteration
In the oxidation zone, oxidation of pyrite forms ferrous (divalent) sulphate and sulphuric acid:
pyrite + oxygen + water → ferric sulphate + sulphuric acid
FeS2 + 7O + H2O → FeSO4 + H2SO4
The ferrous (divalent) sulphate readily oxidizes to ferric (trivalent) sulphate and ferric hydroxide:
ferrous sulphate + oxygen + water → ferric sulphate + ferric hydroxide
6FeSO4 + 3O + 3H2O → 2Fe2(SO4)3 + 2Fe(OH)3
Ferric sulfate is a strong oxidizing agent; it attacks galena as below.
Anglesite and cerussite do not usually occur together. Generally anglesite is stable in lower pH (more acid) environments and cerussite in higher pH (more alkaline) environments. Seawater has a pH of approximately 8.3 (somewhat alkaline) so cerussite is the stable lead supergene mineral in contact with seawater (JRS 18.9,11).
Hydrocerussite requires an alkaline environment, and it cannot co-exist with anglesite (JRS 18.11).
galena, ferric sulphate, water and oxygen to anglesite, ferrous sulphate and sulphuric acid
PbS + Fe2(SO4)3 + H2O + 3O → PbSO4 + 2FeSO4 + H2SO4
Galena is oxidised to anglesite and ferric iron is reduced to ferrous iron (AMU b3).
galena and oxygen to anglesite
In air, at outcrops of galena,
PbS + 2O2 → PbSO4
At ordinary temperatures the equilibrium is displaced far to the right, and the apparent stability of galena is a result of the slowness of the oxidation (KB).
galena may also dissolve in carbonic acid from percolating rainwater to form hydrogen sulphide, which is then oxidised to form anglesite. (KB).
galena and carbonic acid to Pb2+, hydrogen sulphide and HCO3-
PbS + 2H2CO3 → Pb2+ + H2S + 2HCO3- (KB).
hydrogen sulphide, oxygen, Pb2+ and HCO3- to anglesite and carbonic acid
H2S + 2O2 + Pb2+ + 2HCO3- → PbSO4 + 2H2CO3 (KB)
Stability
The Activity-pH diagram below was calculated for some lead minerals. Boundaries are calculated for constant activity (roughly equivalent to concentration) of (SO4)2- and constant partial pressure (also roughly equivalent to concentration) of CO2, over a range of values of pH and of Cl1- activity. In this case the concentration of CO2 is is appreciably more than the atmospheric value.
Anglesite is stable in an acid environment with a low concentration of Cl- ions. If the concentration of CO2 decreases the stability field of anglesite does not change significantly (JRS 15.20).

The lead mineral formulae are:
cotunnite PbCl2
phosgenite Pb2(CO3)Cl2
cerussite Pb(CO3)
anglesite Pb(SO4)
The Activity-pH diagram below is similar, but the concentration of CO2 is close to zero, at about 0.01% of the atmospheric value, and the (SO4)2- activity is about 0.5% of its value in the first diagram.
Cerussite does not form in these conditions, the stability field of mendipite is very large, and mereheadite and plumbonacrite can form, although they are not stable at higher levels of concentration of CO2 (JRS 15.22).
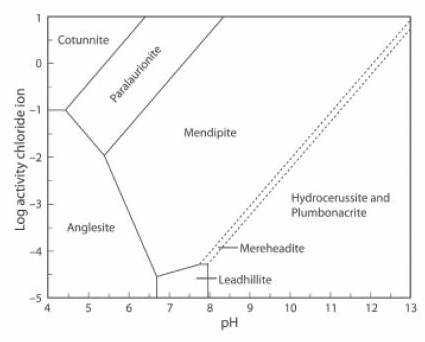
The lead mineral formulae are:
cotunnite PbCl2
paralaurionite PbCl(OH)
mendipite Pb3O2Cl2
mereheadite Pb47O24(OH)13cl25(BO3)2(CO3)
hydrocerussite Pb3(CO3)2(OH)2
plumbonacrite Pb5(CO3)3O(OH)2
anglesite Pb(SO4)
leadhillite Pb4(CO3)2(OH)2
Back to Minerals