Localities
The Two Mile and Three Mile deposits, Paddy's River, Paddys River District, Australian Capital Territory, Australia, are skarn deposits at the contact between granodiorite and volcanic rocks. covellite is a supergene sulphide that occurs as a replacement product of bornite and chalcopyrite, and as coatings along fractures in sulphide-bearing skarn (AJM 22.1.41).
At the Mount Kelly deposit, Gunpowder District, Queensland, Australia, the deposit has been mined for oxide and supergene copper ores, predominantly malachite, azurite and chrysocolla. The ores overlie primary zone mineralisation consisting of quartz-dolomite-sulphide veins hosted in dolomite-bearing siltstone and graphitic schist.
Covellite is the major supergene mineral, occurring as coatings on chalcopyrite crystals, or replacing them, and also as grains and coatings on pyrite (AJM 22.1.20).
At the Mount Lyell mines, Queenstown district, West Coast municipality, Tasmania, Australia, covellite has been found with tenorite (AJM 21.2.24).
At Mount Moliagul, Moliagul, Central Goldfields Shire, Victoria, Australia, covellite sometimes occurs as thin coatings on chalcopyrite (AJM 21.1.42).
At Herrensegen, Schapbach, Schartzwald, Germany, covellite occurs with chalcopyrite (FM 2318).
Alteration
Covellite may occur as an alteration product of chalcopyrite (AJM 18.2.26).
Supergene covellite is formed by the process
Cu2+ + HS- → CuS + H+
Covellite requires an acid environment for stability.
Oxidation of pyrite forms ferrous (divalent) sulphate and sulphuric acid:
pyrite + oxygen + water → ferrous sulphate + sulphuric acid
FeS2 + 7O + H2O → FeSO4 + H2SO4
The ferrous (divalent) sulphate readily oxidizes to ferric (trivalent) sulphate and ferric hydroxide:
ferrous sulphate + oxygen + water → ferric sulphate + ferric hydroxide
6FeSO4 + 3O + 3H2O → 2Fe2(SO4)3 + 2Fe(OH)3
Ferric sulphate is a strong oxidizing agent and attacks both chalcocite and covellite.
chalcocite and ferric sulphate to copper sulphate, ferrous sulphate and covellite
Cu2S + Fe2(SO4)3 → CuSO4 + 2FeSO4 + CuS
(AMU b3-3.7)
covellite and ferric sulphate to ferrous sulphate, copper sulphate and sulphur
CuS + Fe2(SO4)3 → 2FeSO4 + CuSO4 + S
Covellite is further oxidised according to the above reaction to form sulphur (AMU b3-3.7).
sphalerite to covellite: Because covellite is less soluble than sphalerite, supergene covellite may form below the zone of oxidation when dissolved copper ions Cu2+ replace zinc ions Zn2+ from sphalerite
Cu2+ + sphalerite → covellite + Zn2+
Cu2+ + ZnS → CuS + Zn2+
(KB p527)
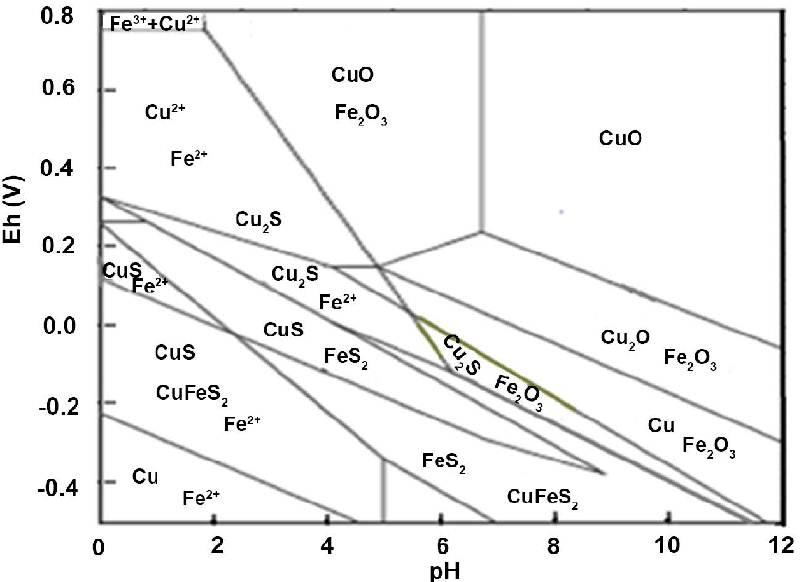
The diagram below is a Pourbaix diagram for Cu-Fe-S-H2O (IJNM 07(02).9.23). It shows the relationship between copper Cu, chalcopyrite CuFeS2, tenorite CuO, covellite CuS, cuprite Cu2O, chalcocite Cu2S, pyrite FeS2 and hematite Fe2O3.
Back to Minerals