It may be found as an oxidation product coating native copper, or as an alteration product of chalcopyrite.
Localities
At the Mount Kelly deposit, Gunpowder District, Queensland, Australia, the deposit has been mined for oxide and supergene copper ores, predominantly malachite, azurite and chrysocolla. The ores overlie primary zone mineralisation consisting of quartz-dolomite-sulphide veins hosted in dolomite-bearing siltstone and graphitic schist.
Cuprite crystals to 2 mm occur, some pseudomorphing chalcopyrite and some encapsulated in calcite. The crystals commonly occur in small cavities and lining fractures in siliceous goethite-hematite rich gossan. The acicular variety chalcotrichite has also been found here (AJM 22.1.21).
Cuprite from Queensland - Image
At Chengmenshan Mine, Jiujiang Count, Jiujiang, Jiangxi, China, cuprite variety chalcotrichite is found as nests of fine red needles (AESS).
Chalcotrichite from Chengmenshan - Image
At the M'sesa mine, Kambove District, Haut-Katanga, DR Congo, shattuckite pseudomorphs after cuprite have been found (KL p231).
At the Mashamba West Mine, Kolwezi mining district, Lualaba, DR Congo, shattuckite pseudomorphs after cuprite occur (R&M 87.4.304-336).
Cuprite from the Mashamba West Mine - Image
At Tsumeb, Namibia, cuprite occurs in dolomite ore, and pseudomorphs of malachite after cuprite have been recorded (R&M 93.6.542).
Cuprite at Tsumeb forms cochineal-red crystals up to about 3 cm in size, as octahedrons, dodecahedrons or some combination thereof, in all three oxidation zones. Some brilliant red octahedral crystals were found with native amalgam, and others were found with rosasite. Hair-like masses of crystals (the variety chalcotrichite) occur, sometimes as red inclusions in minerals such as calcite and cerussite. Pseudomorphs of bayldonite after octahedral cuprite crystals are also known (Minrec 55.6 supplement p92).
Cuprite from Tsumeb - Image
At the Rubtsovsk mine, Rubtsovsky District, Altai Krai, Russia, copper pseudomorphs after cuprite have been found (R&M 95.3.275).
Cuprite from Rubtsovsk - Image
At the Bardon Hill quarry, Coalville, Leicestershire, England, UK, cuprite occurs with native copper altering to chrysocolla and malachite (RES p193).
At New Cliffe Hill quarry, Stanton under Bardon, Leicestershire, England, UK, cuprite occurs with native copper and malachite (RES p197).
Cuprite from the New Cliffe Hill Quarry - Image
At the Cotopaxi Mine, Colorado, USA, cuprite crystals are found rimmed with green malachite. (R&M 84.6.547).
The Central Mine, Central, Keweenaw county, Michigan, USA, initially targeted a series of sub-parallel mineralised fissure veins where the most copper-rich portion of the vein was close to the base of the main greenstone flow.
Cuprite from the Central mine occasionally forms thin veinlets and masses in rich parts of the mineralised fissure veins, but is mainly known as the source of fine deep red patinas sometimes seen on copper specimens, particularly from early finds in the 19th century (MinRec 54.1.53-81).
At the Copper Falls Mine, Copper Falls, Keweenaw county, Michigan, USA, mineralisation occurs primarily in hydrothermal veins cutting preexisting lavas and as amygdules in the Ashbed flow.
Cuprite occurs as oxide coatings on native copper, imparting a distinctive reddish patina. Native copper specimens coloured reddish by cuprite are among the most desirable of all specimens from this mine. In addition, specimens have been found of the acicular “chalcotrichite” variety with needles measuring as much as 1 cm in length (MinRec 54.1.107).
The Cliff Mine, Phoenix, Keweenaw county, Michigan, USA, is situated at the base of a roughly 70-metre basalt cliff. A curious feature of the impressive thickness of the greenstone flow here is that it contains zones of “pegmatoid”: areas where slow cooling in the core of the lava flow allowed for large feldspar crystals exceeding 1 cm to grow. Such features are normally only observed in intrusive igneous rocks and are almost unheard of in basalt flows.
The Cliff mine primarily exploited rich copper mineralisation in the Cliff fissure (vein). Although mineralised with copper to some extent along its entire length, the part of the vein just below the greenstone flow carried the richest copper mineralisation by far. A significant amount of the copper recovered at the Cliff mine came from amygdaloids in the tops of 13 basalt flows which were cut by the Cliff vein. The discovery and mining of this vein proved that the veins were the source of the large masses of float copper that were already well known, and proved that the primary ore mineral in the district was native copper, not sulphides, as had been suspected earlier.
Although cuprite is most common as a red oxide coating on copper, the Cliff mine also produces occasional dark red microcrystals to 2 mm showing octahedrons, cubes and the very rare gyroid (MinRec 54.1.25-49).
Cuprite from Keweenaw County - Image
At the Chino mine, New Mexico, USA, cuprite occurs overgrown with goethite. (R&M 84.6.492-500).
Cuprite from the Chino Mine - Image
The Mufulira Mine, Mufulira, Mufulira District, Copperbelt Province, Zambia, has produced some of southern Africa’s finest cuprite crystals. Most of them are simple octahedrons or cuboctahedrons, often with slightly hoppered faces, and associated with native copper. Beautiful specimens of octahedral cuprite crystals perched on arborescent native copper were commonly collected in the oxide zones at Mufulira West. Cuprite also occurs more rarely as metallic red crystalline masses associated with quartz veins in intensely oxidised areas of the orebody. These masses are usually vuggy and contain cavities lined with malachite. Some cuprite crystals occur perched on pinkish saddle-shaped dolomite crystals (MinRec 55.4.465).
Cuprite from Mufulira - Image
Alteration
chalcocite, oxygen and water to cuprite and sulphuric acid
If acidic copper sulphate solutions pass through the oxidation zone to below the water table, conditions usually change to reducing and the dissolved copper ions react with sulphide ions to form copper sulphides such as chalcocite. If the water table falls, allowing the chalcocite to be exposed to the oxidation zone, then cuprite may be formed according to the above equation (JRS 18.14).
The diagram below is a Pourbaix diagram for copper (GSJ). It shows the relationship between copper Cu, cuprite Cu2O and spertiniite Cu(OH)2.

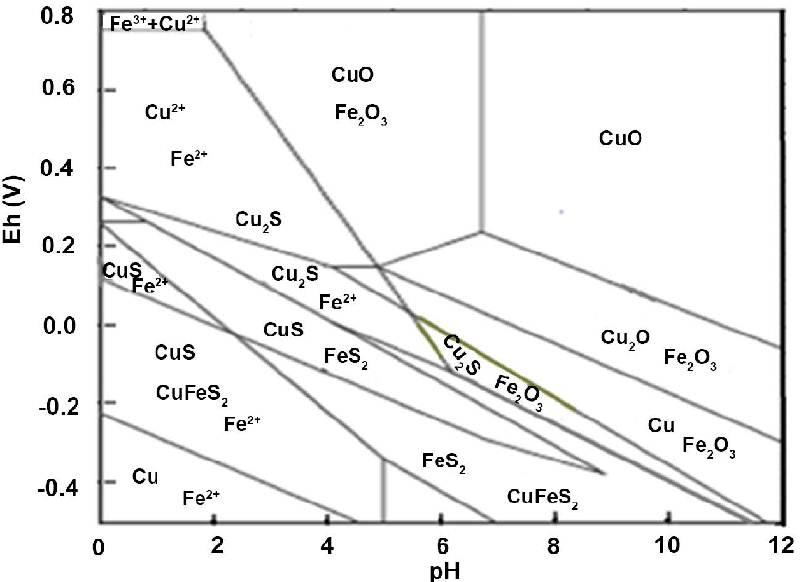
The diagram below is a Pourbaix diagram for Cu-Fe-S-H2O (IJNM 07(02).9.23). It shows the relationship between copper Cu, chalcopyrite CuFeS2, tenorite CuO, covellite CuS, cuprite Cu2O, chalcocite Cu2S, pyrite FeS2 and hematite Fe2O3.
Back to Minerals